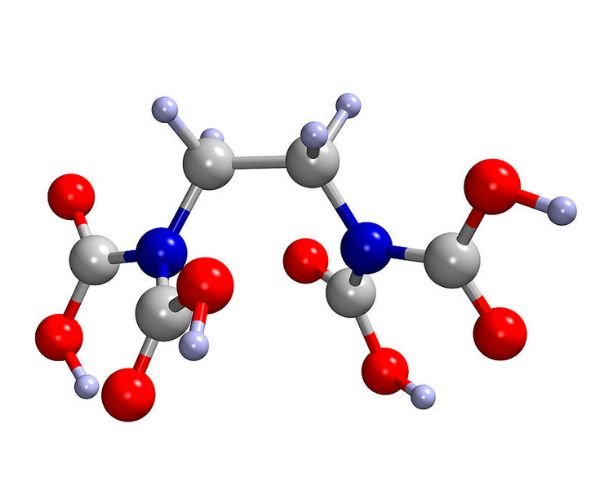
Ethylenediaminetetraacetic acid (EDTA) is one of the most widely used chelating agents in industrial production and agricultural applications. Owing to its strong chelating ability, EDTA can effectively “bind and lock” metal ions, thereby modifying their chemical properties and improving their availability or mobility. Structurally, EDTA contains two amino groups and four carboxyl groups, each carrying lone pair electrons that form coordination bonds with metal ions. This multidentate configuration ensures that EDTA-metal complexes are generally very stable.
However, the stability of EDTA complexes is strongly influenced by pH conditions. Under acidic or alkaline environments, chelated metal ions may be released or precipitated, which is highly relevant for fields such as water treatment, soil management, and fertilizer applications.
1. Calcium and Magnesium Chelates under Acidic Conditions
EDTA-calcium (Ca-EDTA) and EDTA-magnesium (Mg-EDTA) are relatively stable at neutral pH. However, under acidic conditions (pH < 4–5), their stability decreases significantly.
- Mechanism: In acidic solutions, hydrogen ions (H⁺) protonate the carboxyl groups of EDTA. This protonation alters the electron distribution and weakens the coordination bonds with Ca²⁺ and Mg²⁺.
- Result: Ca²⁺ or Mg²⁺ ions are gradually released from the chelate, leading to partial dissociation.
- Practical implication: In water treatment, when Ca-EDTA or Mg-EDTA complexes are present, acidification can release calcium and magnesium ions back into solution, facilitating subsequent removal or processing.
2. Iron Chelates under Alkaline Conditions
The stability of EDTA-iron (Fe-EDTA) is highly dependent on solution alkalinity.
- Mechanism: At high pH (above 8–9), iron ions undergo hydrolysis. Hydroxide ions (OH⁻) combine with Fe³⁺ to form insoluble iron hydroxide [Fe(OH)₃].
- Result: Precipitation of Fe(OH)₃ breaks down the chelation, reducing the availability of Fe-EDTA.
- Practical implication: In alkaline soils, Fe-EDTA (and similar chelates such as Al-EDTA) may precipitate, limiting micronutrient uptake by plants and potentially causing deficiencies.
3. Zinc Chelates under Extreme pH Conditions
EDTA-zinc (Zn-EDTA) demonstrates instability under both extremely acidic and strongly alkaline conditions.
- Strongly acidic conditions (pH < 3–4): Excess hydrogen ions cause intense protonation of EDTA, disrupting the chelate structure and releasing Zn²⁺.
- Strongly alkaline conditions (pH > 10–11): Zn²⁺ reacts with OH⁻ to form insoluble zinc hydroxide [Zn(OH)₂], which precipitates from solution and destabilizes the Zn-EDTA complex.
Conclusion
The stability of EDTA-metal complexes is not constant, but rather strongly influenced by the surrounding pH environment:
- Acidic conditions destabilize Ca-EDTA and Mg-EDTA.
- Alkaline conditions destabilize Fe-EDTA and Al-EDTA.
- Extreme pH conditions (both low and high) destabilize Zn-EDTA.
For industries and agriculture, understanding these mechanisms is crucial. Whether in water treatment, soil conditioning, or fertilizer formulation, managing pH levels is essential to maintain chelate stability and ensure the effective utilization of nutrients.